Real Info About How To Increase Reaction Rate
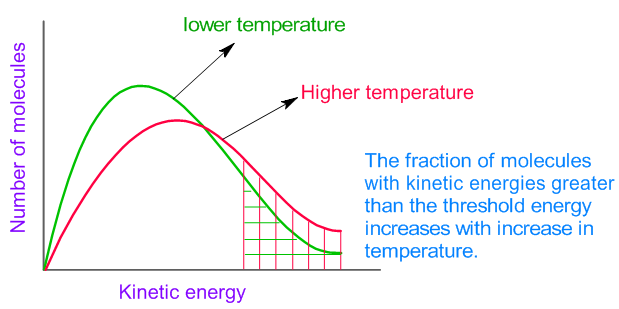
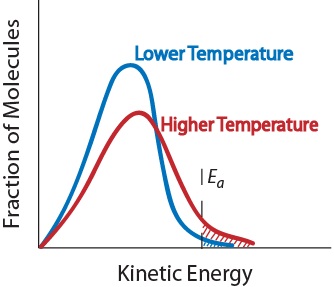
Increasing temperature means the molecules move faster.
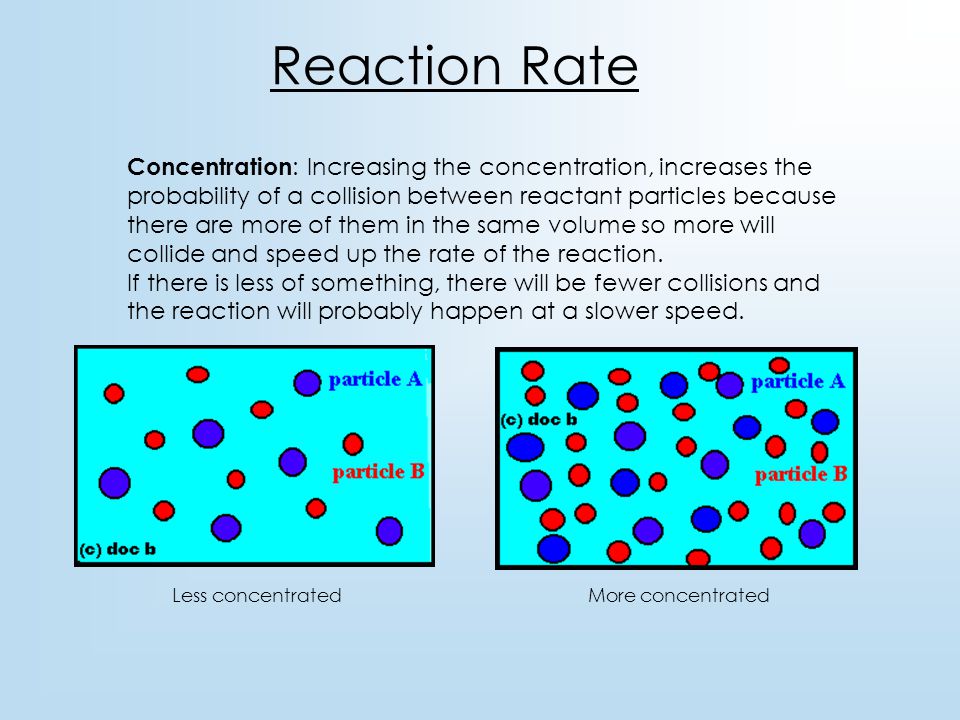
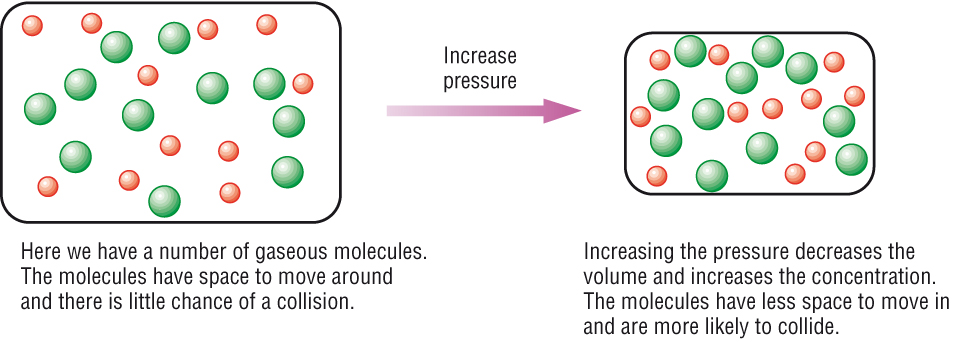
How to increase reaction rate. More movement = more chances of collision. Smaller space = more chances of collision. Typically, increased concentrations of reactants increases the speed of the reaction,.
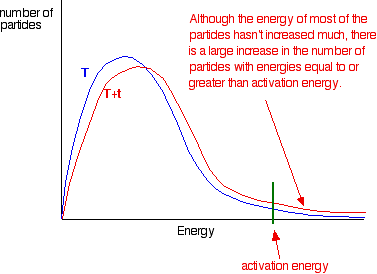
The reaction rate increases in the direction of less gaseous molecules and decreases. Here, a is a constant for the frequency of particle collisions, ea is the activation energy of the reaction, r is the universal gas constant, and t is the absolute temperature. An increase in temperature typically increases the rate of reaction.
An increase in temperature will raise the average kinetic energy of the reactant molecules. The rate law uses the molar concentrations of reactants to determine the reaction rate. There are 4 methods by which you can increase the rate of a reaction:
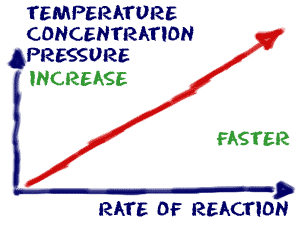
In general, increasing the concentration of a reactant in solution, increasing the surface area of a solid reactant, and increasing the temperature of the reaction system will all increase the rate. Increase the concentration of a reactant. This is due to the.
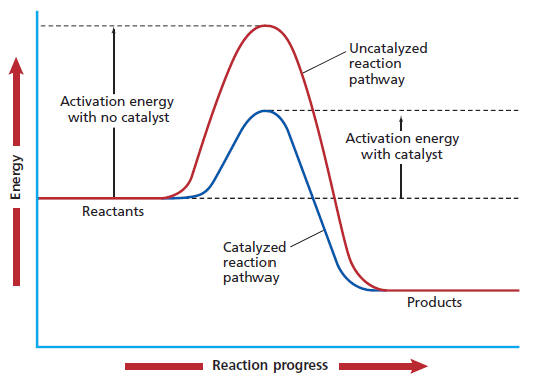
Increase the temperature of the reactants. This is because in each case an. In general, anything that increases the number of collisions between particles will increase the reaction rate, and.
There are 4 methods by which you can increase the rate of a reaction: Concentration (of solutions) surface area, concentration and pressure all have the same effect on reaction rate (an increase leads to a faster reaction rate). How can you increase reaction rate?
More collisions afford more opportunities for reaction. Pressure increases the concentration of gases which in turn results in the increase of the rate of reaction. Increase the concentration of a reactant.
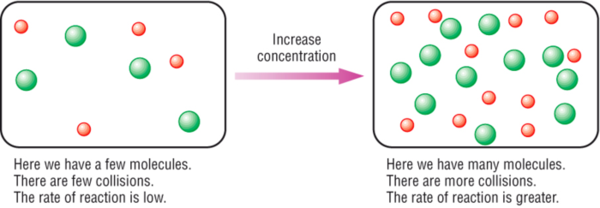
Reaction rate increases with concentration of reactant. Hence, rate of reaction at a given time = gradient of the curve at that instant. 17.5 “temperature and reaction rate”).
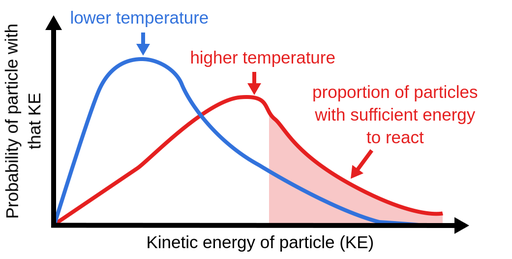
Increasing temperature also means the molecules are moving around faster and will therefore bump into each other more often. The rate of reaction at a given time, t, can be calculated through the following steps. Therefore, a greater proportion of molecules will have the minimum energy necessary for an effective collision (figure.
As concentration of reactants increases, the rate of the reaction will increase. From the arrhenius equation, it is apparent that temperature is the main factor that affects the rate of a chemical reaction. By heating the mixture, you are raising the energy levels of the molecules involved in the reaction.